Ionic liquids: a spectroscopic investigation
Ionic liquids (IL) are salts formed by organic cations, like imidazolium, pyrrolidinium, ammonium or alkyl phosphonium, and organic/inorganic anions, like hexafluorophosphate, tetrafluoroborate, triflate, dicyanamide, tetracyanamethanide or bis(trifluoromethanesulfonyl)imide (TFSI). The presence of such bulky and asymmetric ions decreases the ion-ion interactions and lowers the melting point with respect to more classical salts, reaching values as low as -20°C.

ILs posses many peculiar properties, such as an extremely low vapor pressure, a high ionic conductivity, a high thermal, chemical and electrochemical stability, a high thermal capacity and a good solvent capability. In view of those properties, ILs have been proposed for a large variety of applications in chemistry and physics, such as, for example, green solvents, electrolytes for electrochemistry, lubricants, ingredients for pharmaceuticals and heat exchangers.
Among all possible applications, the use of ILs in electrochemistry as solvents for replacing high flammable and volatile carbonates in lithium batteries is one of the most challenging. In this context, we have recently investigated the thermal properties of a ionic liquid, 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR14 – TFSI), swelling a polyvinylidene?uoride (PVdF) electrospun membrane which has been proposed as an innovative separator in lithium batteries. This study was conducted in the temperature range between 170 and 300 K by means of differential scanning calorimetry (DSC) and dynamical mechanical analysis (DMA). DSC and DMA measurements indicate that the pure ionic liquid transforms into a glass around 190 K. On heating, the glass transforms into a supercooled liquid and around 217 K it crystallizes. On further heating a solid-solid phase transition is revealed around 241 K and finally the sample melts at ~ 268 K. This thermal behavior is displayed also by PYR14 – TFSI swelling an electrospun PVdF membrane, when the cooling process is conducted with a temperature rate of 4 K/min. However, we reported for the first time that when the temperature rate is slower (0.5 K /min) the IL crystallizes during the cooling process around 215 K, possibly due to the interaction with the electrospun polymer membrane.
In order to further investigate the possible interactions of PYR14 – TFSI with the PVdF electrospun membrane, we performed a detailed investigation of the pure IL and of the IL swelling the polymeric membrane as a function of temperature by means of infrared spectroscopy measurements.
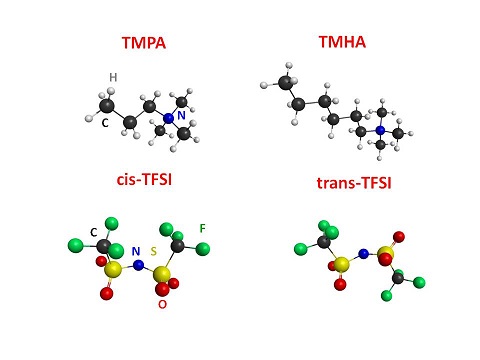
Moreover we studied the infrared absorption spectra of two ionic liquids with bis(trifluoromethanesulfonyl)imide (TFSI) as anion and ammonium with different alkyl chains as cations are reported as a function of temperature. Using the comparison with ab-initio calculations of the infrared active intramolecular vibrations, the experimental lines were ascribed to the various ions composing the ionic liquids. In the liquid state of the samples, both conformers of the TFSI ion are present. In the solid state, however, the two conformers survive in N-trimethyl-N-propylammonium bis(trifluoromethanesulfonyl)imide (TMPA-TFSI), while only cis-TFSI is retained in N-trimethyl-N-hexylammonium bis(trifluoromethanesulfonyl)imide (TMHA-TFSI). We suggested that the longer alkyl chains of the former compound stabilize the less stable conformer of TFSI by means of stronger interactions between anions and cations.
REFERENCES
1) F. M. Vitucci, D. Manzo, M. A. Navarra, O. Palumbo, F. Trequattrini, S. Panero, P. Bruni, F. Croce, A. Paolone
Low temperature phase transitions of 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide swelling a PVdF electrospun membrane
Journal of Physical Chemistry C 118, 5749 (2014)
2) F. M. Vitucci, F. Trequattrini, O. Palumbo, J.-B. Brubach, P. Roy, A. Paolone
Infrared spectra of bis(trifluoromethanesulfonyl)imide based ionic liquids: experiments and DFT simulations
Vibrational Spectroscopy 74, 81 (2014)
3) F. M. Vitucci, F. Trequattrini, O. Palumbo, J.-B. Brubach, P. Roy, M. A. Navarra, S. Panero, A. Paolone
Stabilization of Different Conformers of Bis(trifluoromethanesulfonyl)imide Anion in Ammonium-Based Ionic Liquids at Low Temperatures
Journal of Physical Chemistry A 118, 8758 (2014)