Solid state hydrogen storage
Hydrogen is attracting renewed interest as an energy carrier, due to the necessity of finding ecological energy media which may decrease the environmental pollution from fossil fuels. Hydrogen storage represents a nodal point for the development of a hydrogen economy.
Of the three possible ways to store hydrogen, i.e. as high pressure gas, as a liquid (~20 K at atmospheric pressure), or as hydrides in solids, the latter one appears as the most promising, due to the high mass and volume density and safety. The development of hydrogen storage media is currently considered as the most technologically challenging way for achieving a hydrogen-based economy. There are many compounds with promising hydrogen absorption capacities which, however, display serious drawbacks, like lack of reversibility, slow kinetics or deterioration with proceeding cycling. There is currently general agreement that adopting only traditional materials and methods will not lead to the achievement of the strict requirements necessary for the solid state hydrogen storage. At present, a considerable part of the international research efforts is devoted to the study of solid state hydrogen storage materials finely dispersed on artificial nanoporous supports. This approach is considered promising, since the use of thin layers of hydrogen absorbing compounds is expected to avoid sample compacting and to increase the surface to volume ratio to very high values. However the real interest in the dispersion of nanoparticles into nanoscaffolds resides in the possibility of obtaining a compound with better storage properties than the starting bulk material.
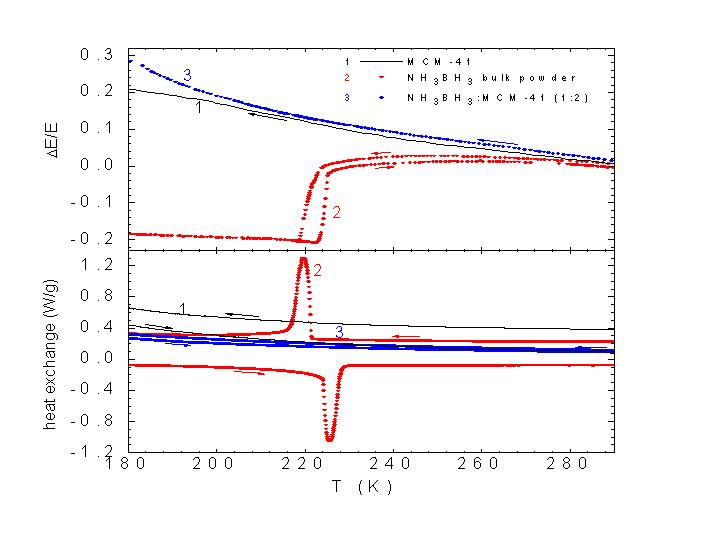
Fig. 1 Effect on the Young modulus of the confinement of ammonia borane into mesoporous silica, MCM-41 [2]

An important task of the research conducted in our Laboratory is the understanding of the basic mechanisms of the hydrogenation/dehydrogenation process and the changes induced by the nanoconfinement. A combined study performed by anelastic spectroscopy and differential scanning calorimetry allowed us to show that one of the most promising compounds, ammonia borane (NH3BH3), finely dispersed in the channels of mesoporous silica does not undergo the structural phase transition present in the bulk, thus providing a clear indication that the basic physical properties of this material are strongly modified by such assembling on a nanoscale. The occurrence of different electronic and lattice interactions in nanostructured scaffolded hydrogen storage systems could provide an alternative approach to modify the thermodynamic features of bulk materials to obtain enhanced dehydrogenation properties and to accomplish reversibility. Finally anelastic spectroscopy has been proven to be a powerful tool, complementary to NMR and neutron scattering, in the study of the hydrogen dynamics. This information is highly demanded, because the hydrogenation/dehydrogenation of storage materials resides on the possibility for hydrogen atoms to perform short or long range diffusion processes. Anelastic spectroscopy investigations conducted in alanates allowed us to propose a model for the dehydrogenation process. The extension of the studies in ammonia borane lead us to identify the rotational and torsional dynamics of H atoms and to derive their activation energies [2]. Moreover, we used the measurements of the dynamic elastic modulus, which is very sensitive to the occurrence of phase transformations, to characterize the nature and the kinetics of the phase transition in NH3BH3 [3].
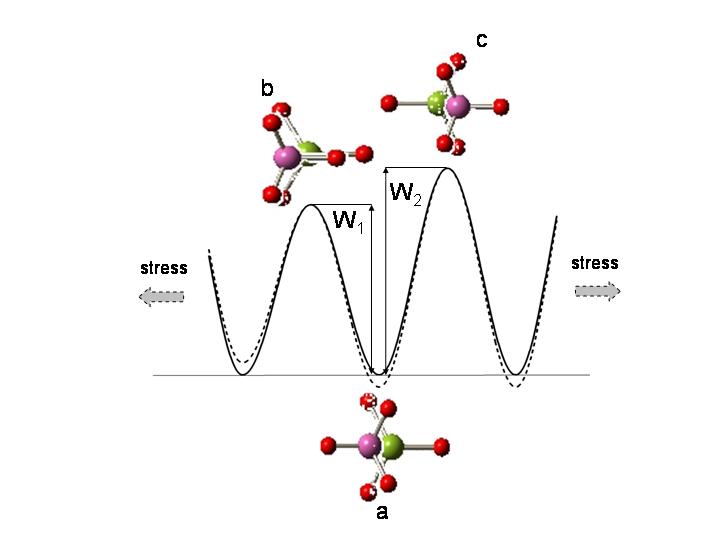
Fig. 2 Schematic representation of the rotational and torsional dynamics of the NH3BH3 molecule with the corresponding energy profile [3].

References
1. O. Palumbo, A. Paolone, R. Cantelli, D. Chandra, Int. J. Hydrogen Energy 33, 3107 (2008).
2. A. Paolone, O. Palumbo, P. Rispoli, R. Cantelli, T. Autrey, J. Phys. Chem C 113, 5872 (2009).
3. A. Paolone, O. Palumbo, P. Rispoli, R. Cantelli, T. Autrey, A. Karkamkar, J. Phys. Chem C 113, 10319 (2009).
4. O. Palumbo, A. Paolone, P. Rispoli, R. Cantelli, T. Autrey, J. Power Sources 195, 1615 (2010)
5. A. Paolone, F. Vico, F. Teocoli, S. Sanna, O. Palumbo, R. Cantelli, D. A. Knight, J. A. Teprovich Jr, R. Zidan, J. Phys. Chem C 116, 16365 (2012)
6. A. Paolone, F. Teocoli, S. Sanna, O. Palumbo, T. Autrey, J. Phys. Chem C 117, 729 (2013)